D. Glen McMaster
Department of Zoology, University of Manitoba
Winnipeg, Manitoba, Canada R3T 2N2
| Delayed hatching of artificially
incubated Brown-headed Cowbird eggs D. Glen McMaster Department of Zoology, University of Manitoba Winnipeg, Manitoba, Canada R3T 2N2 |
 |
The incubation period of avian eggs may be influenced by the behaviour of the incubating parent (Ricklefs and Smeraski 1983; Briskie and Sealy 1990), thermal properties of the nest (Schaeffer 1980; Ricklefs and Smeraski 1983), and physical properties of the egg itself such as mass and shell thickness (O’Connor 1984). Interspecific variation in incubation periods also occurs independently of variables such as egg mass, likely reflecting species-specific differences in rates of embryonic growth and development (Ricklefs 1993). Longer incubation periods may reduce the amount of time a young bird has to fledge, molt, or accumulate fat reserves for migration, as well as extending its period of vulnerability to nest predators (Perrins 1977; Webb 1987). Therefore, short incubation periods are thought to be desirable for most bird species.
The Brown-headed Cowbird (Molothrus ater, hereafter cowbird) parasitizes passerine hosts with a wide range of body sizes. Nestlings of avian brood parasites must compete with host nestlings for parental care (Redondo 1993). Some parasites eliminate competition by ejecting host eggs and young, or by killing them, e.g. parasitic cuckoos and honeyguides, respectively (Payne 1977). Cowbirds have not evolved such drastic strategies, rather, their nestlings compete with host nestlings for food provisioned by the foster parents, often so successfully that some or all host nestlings starve (Briskie and Sealy 1987; Weatherhead 1989). Cowbird eggs often hatch before host eggs (Nice 1937; Hann 1937; Hofslund 1957; Nolan 1978; McMaster, unpubl. data), and the young gain competitive ‘head starts’ over host nestlings (Mayfield 1992). Recent research has shown that parasitic cowbirds have short incubation periods relative to nonparasitic icterines (Briskie and Sealy 1990), and instances of 10-day incubation periods have been confirmed for 3 species of parasitic cowbirds (Wiley and Wiley 1980; Carter 1986; Briskie and Sealy 1990). The mechanism that enables cowbird eggs to hatch earlier than their hosts is not known.
Four hypotheses have explained the short incubation periods of parasitic cowbirds: (1) cowbird embryos develop more rapidly (Friedmann 1927), (2) female cowbirds retain their eggs in the oviduct for up to 24 hours, thereby allowing the embryo to develop in the female prior to being laid (Hoffman 1929), (3) because incubation periods increase as a function of egg volume (Vleck and Vleck 1987), female cowbirds lay small eggs relative to their body mass to minimize their incubation periods (Briskie and Sealy 1990), (4) female cowbirds invest less energy per egg than expected by mass, forcing the embryo to hatch earlier when it runs out of yolk reserves (G. Kattan, unpubl. data).
Hypotheses #1 and #3 have not received support in the Shiny Cowbird (Molothrus bonariensis) (G. Kattan, unpubl. data), and the Brown-headed Cowbird (Briskie and Sealy 1990), respectively. Using the Shiny Cowbird, Kattan (unpubl. data) found support for Hypothesis #4. However, for species with short incubation periods energy investment per unit egg mass is expected to be relatively low, because the maintenance functions of the embryo are fueled for shorter periods than in species with long incubation periods (Ricklefs 1993). Also, Ankney and Johnson (1985) determined the energy investment in Brown-headed Cowbird eggs did not differ significantly from that predicted by egg mass. Although Hypothesis #2 may apply to parasitic cuckoos that lay eggs every 48 hours (Liversidge 1961), the only evidence supporting the hypothesis in cowbirds appears to have been a single case of an egg-bound female (Nice 1954).
In 1994, I tested whether Brown-headed Cowbird embryos develop more rapidly than host embryos (Hypothesis #1) by artificially incubating eggs of cowbirds and two host species. Artificial incubation in an incubator permits determination of incubation periods under constant conditions of temperature, and humidity. In the incubator it is possible to control any effect differences in egg size, female attentiveness, nest insulation, and ambient temperature may have on egg temperature. Hypothesis #1 predicts that cowbird eggs will have shorter incubation periods in the incubator than host eggs. I also predicted that due to the constant temperature of the incubator, cowbird incubation periods would approximate the shortest incubation period observed under natural conditions (10 days, Briskie and Sealy 1990).
Freshly laid cowbird eggs were obtained from host nests at Delta Marsh in May and June, 1994. Red-winged Blackbird (Agelaius phoeniceus) and Yellow Warbler (Dendroica petechia) eggs were also obtained fresh on the day they were laid, and in all cases were either the first or second egg laid in the clutch. Eggs were relocated to the University Field Station where they were labelled, and the egg length and width measured using dial calipers (egg mass was not recorded). Before being placed in the incubator, most eggs were candled to verify no detectable embryonic development had occurred prior to collection. Eggs were placed in random positions inside the incubator, with 2 - 3 cm separating each egg. The incubator was home-made, consisting of a 0.75 x 0.75 m plywood frame, insulated with 8 cm of styrofoam. A YSI temperature controller maintained the air temperature inside the incubator at 37.5 ± 0.1°C. Relative humidity was maintained at 50 - 60% by filling a large pan in the bottom of the incubator with water. Three electric fans located at different levels of the incubator provided continuous air circulation, without blowing directly on the eggs. Eggs were turned four times daily to prevent membranes from adhering to the shells. Eggs were candled every 3 - 4 days throughout incubation to monitor embryonic development. Once an egg neared hatching, a cardboard ring was placed around it to ensure once the embryo hatched it could be identified by the presence of its labelled eggshell. The incubator was checked for hatchlings at least 4 times a day. Newly hatched birds were weighed, and the time of their discovery recorded. The hatching event was observed directly in many instances, allowing perfect accuracy of hatching time. However, if the hatching event was not observed, the hatching time was estimated to be the midpoint between the time the nestling was discovered and the time of the last visit to the incubator.
Egg volumes were calculated using the formula V = kLB2 (where k = 0.515 for cowbirds, k = 0.49 for warblers, L = length of the egg, B = breadth of the egg) (Hoyt 1979; Mills 1987). The data were tested for normality using a Shapiro-Wilk test. Normally distributed variables were analyzed using standard parametric techniques.
Hatching success was similar for both cowbird and warbler eggs, however, Red-winged Blackbird eggs experienced poor hatching success (Table 1). Of the three species, only cowbird eggs showed no embryonic development (10.6% of eggs incubated). Death of embryos while hatching occurred infrequently.
| Table 1. Percent hatching success, embryonic death, sterility and hatching death experienced by artificially incubated eggs of cowbirds and two host species. | |||||
| Species | No. Eggs | % Match Success (n) | % Embryonic Death (n) | % Sterile (n) | % Hatching Death (n) |
| Cowbird | 61 | 60.7 (37) | 24.5 (15) | 11.4 (7) | 3.2 (2) |
| Yellow Warbler | 19 | 52.6 (10) | 47.4 (9) | --- | --- |
| Red-winged Blackbird | 10 | 10.0 (1) | 80.0 (8) | --- | 10.0 (1) |
Cowbird and Yellow Warbler incubation period (W = 0.96, p = 0.28; W = 0.97, p = 0.85, respectively), and egg volume (W = 0.95, p = 0.20; W = 0.84, p = 0.0473, respectively) were normally distributed. Mean egg volumes and incubation periods for each species are shown in Table 2. The sample size for Red-winged Blackbird incubation period was too small to allow further analysis. A 2 sample t-test for groups with unequal variance indicated that the mean incubation period for Yellow Warblers was significantly shorter than that for cowbirds (t = 3.82, df = 29, p = 0.0007). Cowbird incubation periods increased with egg volume (Fig. 1; y = 15041.8 + 0.94(egg vol.), r2 = 0.098), a trend that approached significance (F = 3.58, p = 0.0674).
| Table 2. Mean egg volume and incubation period for artificially incubated cowbird and host eggs that hatched successfully. | ||
| Species | Mean Egg Volume (cm3) ± SE (n) | Mean Incubation Period (D:H:M ± H:M) (n) |
| Cowbird | 2.99 ± 0.04 (35) | 12:10:25 ± 2:14 (37) |
| Yellow Warbler | 1.32 ± 0.04 (10) | 11:22:25 ± 2:13 (10) |
| Red-winged Blackbird | 3.73 ± 0.13 (2) | 12:12:0 ± 1:0 (2) |
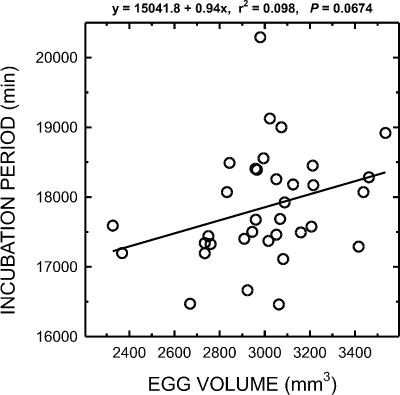
Figure 1. Cowbird incubation period
(minutes) as a function of cowbird egg volume (mm3).
16,500 min = approximately 11.5 days; 18,000 min = 12.5 days.
Hatching success of artificially incubated eggs was lower than that observed under natural conditions at Delta Marsh (Yellow Warblers: 94.9% hatching success; Cowbirds: 75.0% hatching success; McMaster unpubl. data). Red-winged Blackbird hatching success was poor. Passerine eggs, including cowbird eggs, have been artificially incubated successfully by several researchers (e.g., Baldwin and Kendeigh 1932; Graber 1955, 3 of 5 cowbird eggs hatched successfully; Wetherbee and Wetherbee 1961; Kattan manuscript; Dufty pers. comm., 14 of 19 cowbird eggs hatched successfully). Baldwin and Kendeigh (1932) suggested the hatching success of passerine eggs could be maximized by mimicking the gradual onset of full incubation behaviour by female passerines; they increased the incubator temperature gradually from 35.0°C to 37.8°C over the first three days of incubation, with a 0.5 hour period of cooling on each of the first 4 days. Despite these procedures, the maximum hatching success obtained by Baldwin and Kendeigh (1932) was only 50%. Other researchers have found certain species of birds (Red-winged Blackbirds, Yellow-headed Blackbirds (Xanthocephalus xanthocephalus) and Northern Cardinals (Richmondena cardinalis) in particular) are difficult to incubate successfully in the lab (Daniel 1957; Wetherbee and Wetherbee 1961; Dufty pers. comm.). Red-winged Blackbird eggs develop normally in the incubator up to the 7th day, or if incubated naturally up to the 7th day and then moved to the incubator, but it is extremely difficult to incubate them through the entire incubation period (Daniel 1957). It may be that fresh passerine eggs are more difficult to incubate successfully than those that have received some natural incubation, however all 5 fresh cowbird eggs incubated by Dufty (pers. comm.) hatched. The temperature and humidity levels in my incubator were virtually identical to those employed by all other researchers for passerine eggs. Therefore, the reason for the poor hatching success in this study is not known.
Cowbird incubation periods in the artificial incubator were much longer than had been predicted. Under natural conditions cowbird eggs hatch either before (45%) or the same day (41%) as Yellow Warbler eggs (McMaster, unpubl. data). Instead of being the first eggs to hatch, cowbirds hatched at approximately the same time as Red-winged Blackbirds, and took significantly more time to hatch than Yellow Warblers. Cowbird incubation periods in the incubator were on average 2.5 days longer than the 10 day incubation period reported by Briskie and Sealy (1990), and were 12.4 hours longer than the mean cowbird incubation period under natural conditions (Briskie and Sealy 1990). However, the long cowbird incubation periods in this study were similar to those obtained by A. Dufty (pers. comm.). Dufty recorded a mean incubation period of 12.6 days for 5 fresh cowbird eggs in an artificial incubator. Other researchers have also incubated cowbird eggs artificially (Graber 1955; Wetherbee and Wetherbee 1961), but their eggs were not freshly laid, so accurate incubation periods were not determined. In contrast to the long incubation periods observed for Brown-headed Cowbirds in the incubator, the mean incubation period obtained by Kattan for Shiny Cowbird eggs in the incubator (11.7 ± 0.5, n = 11) was shorter than that observed in House Wren (Troglodytes aedon) nests (12.0 ± 0.8, n = 7). However, Shiny Cowbird incubation periods obtained by Kattan in the incubator were still significantly longer than the 10.0 day incubation periods observed for this species by Wiley and Wiley (1980). Due to the difference in cowbird incubation periods observed between natural and artificial conditions, one must question whether artificial incubators simulate natural conditions well enough to be used in studies of incubation periods. Evidence to the contrary is found in the fact that host eggs in this study had incubation periods similar to eggs incubated naturally (McMaster unpubl. data), as well as artificially by other researchers. Red-winged Blackbird eggs hatched successfully in artificial incubators have taken 12 - 12.5 days (Daniel 1957; Dufty pers. comm.). Wetherbee and Wetherbee (1961) note the longest incubation period observed for Yellow Warblers was 11.125 days, which they say appeared to be too short to be representative when compared to non-parulides, but they believed the eggs were fresh when collected. Nolan (1978) recorded an incubation period of 11.9 days for a freshly laid Prairie Warbler (Dendroica discolor) egg in an incubator.
Potential sources of error which could have influenced the rate of embryonic development in the incubator include; (1) temperature and humidity were optimal for the Red-winged Blackbird and Yellow Warbler eggs, but suboptimal for the cowbird eggs, (2) host eggs received more incubation prior to being placed in the incubator, and (3) cowbird eggs were in areas of the incubator where temperature gradients resulted in less rapid cowbird development. Neither temperature or humidity were likely suboptimal for cowbirds, given they had similar hatching success to Yellow Warblers, and much greater hatching success than Red-winged Blackbirds. Indeed, given that cowbirds are generalist brood parasites whose eggs are laid in many species of birds nests (over 200 species, Payne 1977), one might expect cowbird eggs to be more tolerant to variable incubation temperature and humidity than other bird species. Groebbels and Mobert (1930) suggest embryos of the brood parasitic Cuckoo (Cuculus canorus) are more resistant to chilling than host embryos. Host eggs are unlikely to have received more incubation prior to collection than cowbird eggs, as only eggs known to be freshly laid were used in this study. Yellow Warbler females spend little time on the nest on the first and second days of egg-laying (McMaster, unpubl. data), so eggs likely received little incubation before being collected. Fans were installed in the incubator with the express purpose of minimizing thermal gradients. Even if a small thermal gradient did exist in the incubator, because the eggs of all three species were randomly distributed within it, cowbird incubation periods were unlikely to have been influenced to a greater extent than eggs of the other species.
While the increase in cowbird incubation period with increasing egg volume approached significance, it is clear that much variation in incubation period exists between eggs of different volumes. Egg mass may be a better predictor of incubation period than egg volume. Kattan used egg mass rather than volume to predict Shiny Cowbird incubation periods, and although he found incubation period was significantly correlated with egg mass, egg mass only explained 44% of the variation in incubation period. It is possible that cowbird incubation periods vary between females, however the maternity of cowbird eggs was not determined in this study.
Cowbird eggs incubated artificially in 1994 had longer incubation periods than under natural conditions, whereas the incubation periods of two host species did not differ from those under natural conditions. Why cowbird egg response to artificial incubaiton differed from that of host eggs is not known. Perhaps cowbird eggs require contact with other eggs for normal incubation. This possibility will be tested in 1995 by comparing the incubation periods of cowbird eggs incubated artificially in contact with clutches of host eggs, with those of cowbird eggs incubated individually.
This project could not have been completed without the help of Diane Beattie, Kim Caldwell, Doug Froese, Sharon Gill, Paula Grieef, David Jones, Janice Lorenzana, and Graham Stinson. Dr. Spencer G. Sealy provided both helpful supervision and valuable cowbird eggs throughout the study. Thanks to the staff at the University Field Station for their hospitality, and to the officers of the Portage Country Club for allowing me to conduct research on their property. This project was funded by an NSERC Postgraduate Scholarship and a Wildlife Society Scholarship (Manitoba Chapter) to myself, and an NSERC Research Grant to Dr. S.G. Sealy.
Ankney, C. D. and Johnson, S. L. 1985. Variation in weight and composition of Brown-headed Cowbird eggs. Condor 87: 296-299.
Baldwin, S. P. and Kendeigh, S. C. 1932. Physiology of the temperature of birds. Cleveland Mus. Nat. Hist. 3: 1-196.
Briskie, J. V. and Sealy, S. G. 1990. Evolution of short incubation periods in the parasitic cowbirds, Molothrus spp. Auk 107: 789-794.
Carter, M. D. 1986. The parasitic behavior of the Bronzed Cowbird in south Texas. Condor 88: 11-25.
Daniel, J. C. Jr. 1957. An embryological comparison of the domestic fowl and the Red-winged Blackbird. Auk 74: 340-358.
Friedmann, H. 1927. A case of apparently adaptive acceleration of embryonic growth-rate in birds. Biol. Bull. 53: 343-345.
Friedmann, H. 1963. Host relations of the parasitic cowbirds. U.S. Natl. Mus. Bull. 233: 1-276.
Graber, R. R. 1955. Artificial incubaiton of some non-galliform eggs. Wilson Bull. 67: 100-109.
Groebbels, F. and Mobert, F. 1930. Ueber die lebensdauer von vogelembryonen und die lebensdauer des kuckucks im ei. Ornith. Monats. 38: 89-90.
Hann, H. W. 1937. Life history of the oven-bird in southern Michigan. Wilson Bull. 49: 145-237.
Hofslund, P. B. 1957. Cowbird parasitism of the Northern Yellow-throat. Auk 74: 42-48.
Hoffman, E. C. 1929. Cowbirds-decoys-incubation period. Bull. N.E. Bird-Banding Assoc. 5: 118-119.
Hoyt, D. F. 1979. Practical methods of estimating volume and fresh weight of bird eggs. Auk 96: 73-77.
Liversidge, R. 1961. Pre-incubation development of Clamator jacobinus. Ibis 103a: 624.
Mayfield, H. F. 1992. Kirtland’s Warbler (Dendroica kirtlandii). IN: The Birds of North America, No. 19 (A. Poole, P. Stettenheim, and F. Gill, eds.). Philadelphia: The Academy of Natural Sciences; Washington , DC: The American Ornithologists’ Union.
Mills, A. M. 1987. Size of host egg and egg size in Brown-headed Cowbirds. Wilson Bull. 99: 490-491.
Nice, M. M. 1937. Studies in the life history of the song sparrow. Trans. Linn. Soc. N.Y.. 2: 1-246.
Nice, M. M. 1953. The question of ten day incubation periods. Wilson Bull. 65: 81-93.
Nice, M. M. 1954. Problems of incubation periods in North American birds. Condor 56: 173-197.
Nolan, V. Jr. 1978. The ecology and behavior of the Prairie Warbler Dendroica discolor. Ornithol. Monogr. 26: 1-595.
O’Connor, R. J. 1984. Incubation. p.37-40. In: The Growth and Development of Birds. J. Wiley and Sons, Toronto. 315pp.
Payne, R. B. 1977. The ecology of brood parasitism in birds. Ann. Rev. Ecol. Syst. 8: 1-28.
Perrins, C. M. 1977. The role of predation in the evolution of clutch size. p.181-191. In: Stonehouse, B., and C. Perrins eds. Evolutionary Ecology. University Park Press, Baltimore. 310pp.
Redondo, T. 1993. Exploitation of host mechanisms for parental care by avian brood parasties. Etología 3: 235-297.
Ricklefs, R. E. 1993. Sibling competition, hatching asynchrony, incubation period, and lifespan in altricial birds. p. 199-276. IN: Current Ornithology, Vol. 11. (D.M. Power ed.). Plenum Publ. Corp., N.Y.
Ricklefs, R. E. and Smeraski, C. A. 1983. Variation in incubation period within a population of European Starling. Auk 100: 926-931.
Schaeffer, V. H. 1980. Geographic variation in the insulative qualities of nests of the Northern Oriole. Wilson Bull. 92: 466-474.
Vleck, C. M. and Vleck, D. 1987. Metabolism and energetics of avian embryos. J. Exp. Zool. Suppl. 1: 111-125.
Weatherhead, P. J. 1989. Sex ratios, host-specific reproductive success, and impact of Brown-headed Cowbird. Auk 106: 258-366.
Webb, D. R. 1987. Thermal tolerance of avian embryos: a review. Condor 89: 874-898.
Wetherbee, D. K. and Wetherbee, N. S. 1961. Artificial incubation of eggs of various bird species and some attributes of neonates. Bird-Banding 32: 141-159.
Wiley, R. H. and Wiley, M. S. 1980. Spacing and timing in the nesting ecology of a tropical blackbird: comparison of populations in different environments. Ecol. Monogr. 50: 153-178.