Brenda J. Hann
Department of Zoology, University of Manitoba
Winnipeg, Manitoba, Canada R3T 2N2
| Response of aquatic invertebrates
to experimental nutrient enrichment of a wetland Brenda J. Hann Department of Zoology, University of Manitoba Winnipeg, Manitoba, Canada R3T 2N2 |
 |
Delta Marsh, a large lacustrine wetland on the southern shore of Lake Manitoba, is bordered with fertile agricultural land and aspen parkland. Fertilizers and pesticides in runoff from these lands and in groundwater are filtered through the marsh, impacting the aquatic environment including the biota. However, the effects of nutrient enrichment of a eutrophic prairie marsh are poorly known. Nutrient additions have been shown to enhance primary productivity in oligotrophic, nutrient-poor wetlands in the Interlake area of Manitoba (Murkin et al. 1991; Murkin et al. 1994; Gabor et al. 1994). Effects on aquatic invertebrate community diversity and abundance are equivocal (Schoenberg 1988; Rader and Richardson 1992; Murkin et al. 1994; Gabor et al. 1994). Grazing and nutrient recycling by zooplankton and macrophyte-associated invertebrates are potential mechanisms for effecting control over primary producers in lakes and wetlands.
This study sought to examine the effects of controlled additions of nutrients to experimental wetland enclosures. Two temporal patterns of additions were employed: (1) pulse additions at two times during the season, to simulate sudden, large loadings such as following heavy rainfall or seasonal flooding, and (2) press additions trickled in at regular, frequent intervals as might occur from groundwater flow. Specifically, we studied the relationship between temporal pattern of nutrient additions and invertebrate abundance and diversity, and between invertebrate communities and their algal food resources. The manipulative experiment (May - August 1994) was designed to examine "bottom-up" influences on the primary producers and aquatic invertebrates in the marsh food web. With differential nutrient enrichment, community composition and abundance of primary producers (phytoplankton, periphyton, metaphyton, and macrophytes) is expected to change, as would associated grazer communities.
We carried out a nutrient enrichment experiment in Blind Channel in Delta Marsh, MB (50░11’N, 98░12’W), a 22,000 ha freshwater wetland on the southern shore of Lake Manitoba. Six enclosures (5 m x 5 m) were constructed using impermeable woven polyethylene curtains supported on floating platforms (see Goldsborough 1991). The bottom margins were weighted with rebar and sunk into the sediments at least 30 cm, thereby isolating the water in the enclosures from the channel. Water depth was less than 1 m throughout the summer, and the enclosed water volume in each enclosure was approximately 20 m3. Fish that had been trapped in the enclosures during installation were removed using commercial minnow traps, set and emptied daily.
Experimental treatments were assigned to enclosures in a semi-random manner so that no replicate enclosures were adjacent or contiguous to each other (Fig. 1). Sampling was initiated 24 May (week 1) and continued weekly until 26 August (week 14). Weeks 1-4 constituted a pre-treatment period, followed by 10 weeks of treatment period. Nutrients were added according to the ratio 7N:1P, nitrogen (N) as NaNO3 and phosphorus (P) as NaH2PO4.2H2O. Additions were made in the press treatment three times per week (Monday, Wednesday, Friday) of the experiment (beginning on 20 June) in 2 replicate enclosures and in the pulse treatment at week 5 (20 June) and week 10 (25 July) of the experiment in 2 replicate enclosures. Equal total nutrients were added to all treatment enclosures by the end of the experiment. No nutrients were added to control enclosures. Each nutrient addition was prepared in the laboratory by dissolving the requisite weight of inorganic chemical in 1L of carbon-filtered water. Prior to application of the nutrient solution to each enclosure the volume was diluted to 10L and sprinkled uniformly over the water surface.
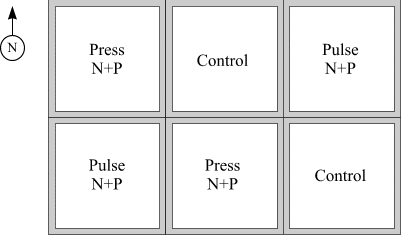
Figure 1. Arrangement of replicated treatments (control, press
and pulse) in 5 m x 5 m enclosures deployed in the Blind Channel, 1994.
Algal communities parameters (biomass as chlorophyll a and productivity) were determined for phytoplankton, periphyton, metaphyton, and epipelon components. Irradiance profiles, turbidity, and water chemistry (including ammonia, orthophosphate, silicon, nitrate, pH, dissolved oxygen, temperature) were monitored during the experiment (see McDougal and Goldsborough 1995 for detailed methods).
Weekly sampling for invertebrates included zooplankton in the water column, zooplankton associated with periphyton, and macrophyte-associated invertebrates. The water column was sampled for zooplankton using a transparent acrylic cylinder 50 cm in length and 5.5 cm in diameter. A 4 L volume was then filtered through a conical net with a mesh size of 80 Ám. Samples were preserved with formalin and the volume of each was standardized to 20 mL. Zooplankton that graze periphyton were sampled using a transparent acrylic cylinder (50 cm x 7 cm) placed around extruded acrylic rods (90 cm x 5 mm) arranged in a 10 x 10 grid in each enclosure. These rods were planted in mid-May and allowed to colonize with periphyton. A 1.6L volume was treated in the same fashion as for zooplankton in the water column. Invertebrates associated with aquatic macrophytes were sampled semi-quantitatively in two ways: (1) using activity traps of a design modified from Whiteside et al. (1978), and (2) using a macrophyte-invertebrate sampler of a design modified from Pip and Stewart (1976). An activity trap consisted of 3 10-cm diameter wide-bore funnels attached to a 20 x 20 cm acrylic plate. Polyethylene sample bottles (125 mL) were secured to the opposite face of the acrylic plate. To set for sampling, activity traps with sample bottles pre-filled with water were lowered through the water column with funnel openings directed downward to rest on macrophytes or sediment. To retrieve, traps were gently raised to the surface using an attached rope, inverted below the water surface, and contents of 3 sample bottles pooled into a 500 mL collection bottle for transport to the laboratory for further processing. Samples were concentrated by passing them through a 80 Ám mesh net, then preserved with 4% formalin. The macrophyte sampler is described in McDougal and Goldsborough (1995).
Zooplankton (Cladocera and Copepoda) were identified to species using various standard references including Pennak (1978), Edmondson (1959), and Smith and Fernando (1978). Rotifers were counted but not identified. Species data are not reported here.
The N and P concentrations in the water column in both press and pulse treatments were found consistently to exceed control levels (McDougal and Goldsborough 1995).
Cladocera predominated in all enclosures throughout the experiment, probably due to fish predator exclusion. Zooplankton in the water column (especially Cladocera) increased in abundance through the season and effectively grazed the phytoplankton crop maintaining it at very low levels.. Mean cladoceran densities for all treatments showed no differential response to experimental nutrient additions (Fig. 2). Copepods and rotifers showed pre-treatment population density peaks, but remained at low density throughout the treatment period (Fig. 3).
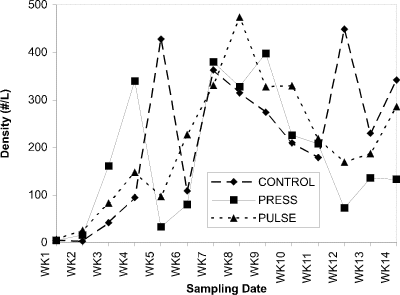
Figure 2. Abundance of cladocerans (no. per liter) in the water
column of control, press and pulse enclosures over a 14-week period.
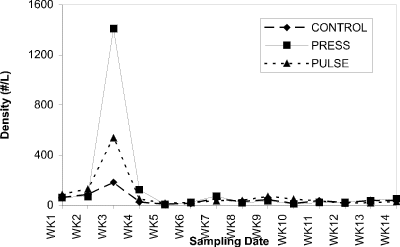
Figure 3. Abundance of cyclopoids (no. per liter) in the water
column of control, press and pulse enclosures over a 14-week
period.
Cladoceran grazers associated with periphyton on acrylic rods (Fig. 4) occurred at densities similar to those in the water column during the pre-treatment period. In response to nutrient additions, their densities increased substantially. Cladoceran densities increased four-fold in response to initial press nutrient additions, then declined by week 9 and remained at pre-treatment levels for the duration of the experiment. Increased cladoceran grazer densities were apparent in response to both pulse nutrient additions (Fig. 4). Copepods grazing periphyton on rods showed a similar response to those in the water column, existing at low densities throughout the treatment period (Fig. 5).
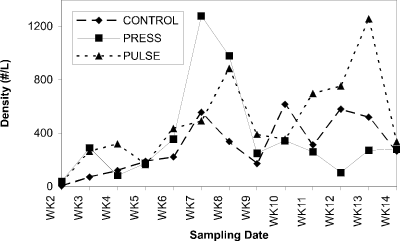
Figure 4. Abundance of cladocerans (no.
per liter) associated with acrylic rods in control, press and
pulse enclosures over a 14-week period.
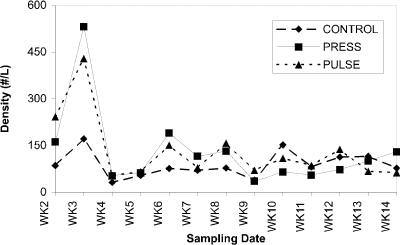
Figure 5. Abundance of cyclopoids (no.
per liter) associated with acrylic rods positioned in control,
press and pulse enclosures over a 14-week period.
Microcrustacean grazers associated with macrophytes increased in abundance during early phases of treatment (week 7-9), in parallel with macrophyte biomass, then declined substantially. Cladocerans responded strongly to the first pulsed nutrient addition (Fig. 6), whereas copepods showed larger increases in abundance in response to press additions (Fig. 7).
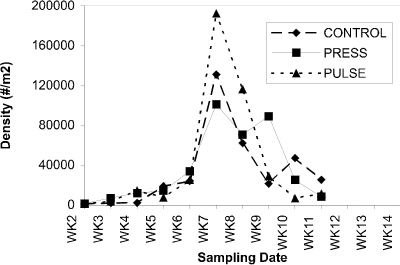
Figure 6. Abundance of cladocerans (no.
per m2) associated
with macrophytes in control, press and pulse enclosures over a
14-week period.
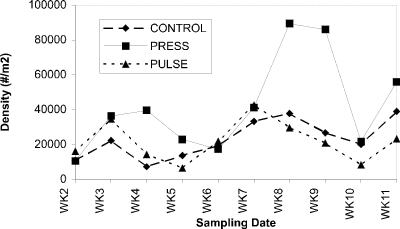
Figure 7. Abundance of cyclopoids (no.
per m2) associated
with macrophytes in control, press and pulse enclosures over a
14-week period.
Metaphyton showed its most pronounced development in the press nutrient treatment, with substantial, though lagged, development in the pulsed treatment (McDougal and Goldsborough 1995). Grazing pressure on metaphyton was not quantified in our study. However, an increase in abundance of the trichopteran, Agraylea multipunctata, which makes its case from filamentous green algae (Wiggins 1977), in samples of the phytophilous invertebrate community was noted.
Invertebrate response to nutrient enrichment of wetlands has been equivocal. Results of preliminary experiments in Florida wetlands showed that nutrient additions generally led to an increase in density of macroinvertebrates (Rader and Richardson 1992). Similarly, water column (=nektonic) herbivores and detritivores (cladocerans, copepods) increased in abundance and biomass in response to inorganic nutrient addition in enclosures in an oligotrophic wetland (Campeau et al. 1994). Epiphytic herbivores and detritivores also increased in abundance and biomass in response to both inorganic and organic (litter) additions (Campeau et al. 1994). When a single large pulsed addition of inorganic or organic (alfalfa) nutrients was made in early spring to two oligotrophic marshes, benthic and nektonic invertebrates showed an increase in abundance in response to the high inorganic treatment. In contrast, Murkin et al. (1994) observed no significant differences in numbers or biomass of total invertebrates or invertebrate functional groups that could be attributed to press fertilization of the same marshes. Our study demonstrated no consistent pattern of invertebrate response to enrichment. However, definitive conclusions must await detailed comparisons between invertebrate densities and the algal/macrophyte biomass within these quite discrete components of the wetland ecosystem.
Due to variable microhabitat preferences and requirements, responses of invertebrates to resource (nutrient) manipulations will differ. Water column microcrustaceans (Cladocera, Copepoda) graze phytoplankton and bacteria (Downing 1981; Schoenberg and Maccubbin 1985), whereas epiphytic species scrape macrophyte surfaces (Downing 1981; Fryer 1968), feeding on attached algae, detritus, and fungi, collectively known as "aufwuchs" (Bowen 1979). Large cladocerans are very efficient grazers and reduced the phytoplankton biomass and primary productivity to low levels despite the nutrient treatments.
Life history characteristics influence the ability of each invertebrate group to respond to changes in food quality and quantity. Cladocerans were the predominant component of the grazing community throughout the treatment phase of the manipulation, in contrast to the pre-treatment phase which was dominated by copepods and rotifers. The exclusion of vertebrate planktivores from the enclosures and the existence of an ephippial (diapause) cladoceran egg bank in the sediments resulted in a large hatch of neonates which, when mature, reproduce parthenogenetically, allowed a rapid response in population size when food resources became available. Conversely, increase in population size of copepods which reproduce exclusively sexually (Pennak 1978) is dependent upon hatching of new cohorts or species from their egg banks over the season. Seasonal distribution and life histories of copepod species are under investigation in Delta Marsh (Zrum and Hann 1995).
Bottom-up experimental manipulation of a wetland food web via nutrient additions has demonstrated a differential response among primary producers and associated invertebrate grazers. Zooplankton grazers effectively depressed phytoplankton biomass in control, press, and pulse enclosures in the absence of fish predators. Zooplankton grazers increased in density in response to increased availability of periphyton on acrylic rods in both press and pulse nutrient treatments, but especially in pulse additions. Macrophyte-associated invertebrates changed in abundance in parallel with macrophyte biomass changes. Metaphyton shading (in both press and pulse treatments) led to macrophyte decline and eventual decomposition, and substantial reduction in phytophilous invertebrate density.
Sampling assistance was provided by Mandy Lloyd, Leanne Zrum, Rhonda McDougal, and Ken Sandilands. The University of Manitoba Field Station supplied logistic support essential to completion of this experiment.
Bowen, S. H. 1979. Determinants of the chemical composition of periphytic detrital aggregate in a tropical lake (Lake Valencia, Venezuela). Arch. Hydrobiol. 87: 166-177.
Campeau, S., Murkin, H. R. and Titman, R. D.1994. Relative importance of algae and emergent plant litter to freshwater marsh invertebrates. Can. J. Fish. Aquat. Sci. 51: 681-692.
Downing, J. A. 1981. In situ foraging responses of three species of littoral Cladocera. Ecol. Monogr. 51: 85-103.
Edmondson, W. T. (ed.) 1959. Freshwater Biology. 2nd Edition. John Wiley and Sons, New York, NY.
Fryer, G. 1968. Evolution and adaptive radiation in the Chydoridae (Crustacea: Cladocera): a study in comparative functional morphology and ecology. Trans. R. Soc. Lond. Ser. B254: 221-385.
Gabor, T. S., Murkin, H. R., Stainton, M. P., Boughen, J. A. and Titman, R. D.. 1994. Nutrient additions to wetlands in the Interlake region of Manitoba, Canada: effects of a single pulse addition in spring. Hydrobiologia 279/280: 497-510.
Goldsborough, L. G. 1991. Effects of hexazinone on water chemistry and periphyton biomass and productivity in large littoral enclosures. University Field Station (Delta Marsh) Annual Report 26: 50-62.
McDougal, R. L. and Goldsborough, L. G. 1995. Responses of wetland algae and macrophytes to press and pulse additions of inorganic nitrogen and phosphorus. University Field Station (Delta Marsh) Annual Report, this volume.
Murkin, H. R., Stainton, M. P., Boughen, J. A., Pollard, J. B. and Titman, R. D. 1991. Nutrient status of wetlands in the Interlake region of Manitoba, Canada. Wetlands 11: 105-122.
Murkin, H. R., Pollard, J. B., Stainton, M. P., Boughen, J. A. and Titman, R. D. 1994. Nutrient additions to wetlands in the Interlake region of Manitoba, Canada: effects of periodic additions throughout the growing season. Hydrobiologia 279/280: 483-495.
Pennak, R. W. 1978. Freshwater Invertebrates of the United States. 2nd Edition. John Wiley and Sons, New York, NY.
Pip, E. and Stewart, J. M. 1976. The dynamics of two aquatic plant-snail associations. Can. J. Zool. 54: 1192-1205.
Rader, R. B. and Richardson, C. J.. 1992. The effects of nutrient enrichment on algae and macroinvertebrates in the Everglades: a review. Wetlands 12: 121-135.
Schoenberg, S. A. 1988. Microcrustacean community structure and biomass in marsh and lake habitats of the Okefenokee Swamp: seasonal dynamics and responses to resource manipulations. Holarctic Ecology 11: 8-18.
Schoenberg, S. A. and Maccubbin, A. E.. 1985. Relative feeding rates on free and particle-bound bacteria by freshwater zooplankton. Limnol. Oceanogr. 30: 1084-1090.
Smith, K. and Fernando, C. H. 1978. A guide to the freshwater calanoid and cyclopoid Crustacea of Ontario. Univ. Waterloo Biol. Ser. No. 18, 74 pp.
Whiteside, M. C., Williams, J. B. and White, C. P.1978. Seasonal abundance and pattern of chydorid Cladocera in mud and vegetative habitats. Ecology 59: 1177-1188.
Wiggins, G. B. 1977. Larvae of the North American caddisfly genera. University of Toronto Press, Toronto, Ont.
Zrum, L. and Hann, B. J. 1995. The impact of invertebrate and vertebrate predation on littoral zooplankton in a wetland ecosystem. University Field Station (Delta Marsh) Annual Report, this volume.