Robert A. Anderson and Reinhart A. Brust
Department of Entomology, University of Manitoba
Winnipeg, Manitoba, Canada R3T 2N2
The frequency at which mosquitoes contact vertebrate hosts is an important aspect of the epidemiology of vector-borne disease (Dye 1992). In most models of disease transmission, it is assumed that there is only 1 host contact per mosquito per gonotrophic cycle, (de Moor and Steffens 1970; Smith 1987). This assumption often is violated because mosquitoes may feed more than once prior to the first batch, or during the period between egg batches. In so doing, they contact more than one host (Edman and Downe 1964; Boreham and Garrett-Jones 1973; Burkot et al. 1988; Anderson et al. 1990; Scott et al. 1993; Anderson and Brust, in press). Thus, it is important to understand factors that influence blood feeding frequency and to estimate accurately the rate of mosquito-host contact.
Multiple meals with blood from distinguishable hosts are defined as patent (Boreham and Garrett-Jones 1973), while those in which serial feeding attempts involve the same individual host or multiple hosts indistinguishable by conventional techniques are defined as cryptic. Boreham and Garrett-Jones (1973) estimated the frequency of cryptic multiple feeding by multiplying the ratio of the probability of a cryptic double meal (the probability of one host being fed on squared) to the probability of a patent double meal (the probability of one host being fed on times the probability of a second host being fed on) by the proportion of detected patent multiple meals. They assumed explicitly that the same hosts are available during both feeds and that the source of each feed is chosen randomly. Burkot et al. (1988) extended the model of Boreham and Garrett-Jones (1973) to include the probability of interruption on each of at least two possible hosts and assumed these probabilities to be equal. However, there is significant variation in defensive behavior of individual hosts, either of the same or different species (Edman et al. 1972; Kale et al. 1972). Consequently, the tendency of vertebrate hosts to interrupt blood feeding mosquitoes may be a more variable phenomenon than assumed by Burkot et al. (1988).
One objective of our paper is to examine the extent to which individual avian hosts vary relative to other individuals of the same species, sex, size and age in the frequency at which they are fed on by 3 species of ornithophilic Culex. Another objective of our paper is to use blood-meal size to estimate and compare the frequency of interrupted blood meals on individual hosts. Additionally, we examine the way in which the frequency of interrupted and multiple feeding by mosquitoes is related to variation in the degree to which individual hosts are fed on by mosquitoes.
Mosquitoes were collected in box traps (Anderson and Brust, in press) at Delta Marsh, Manitoba in 1991, at Vero Beach, Florida in 1992 and at Winnipeg, Manitoba in 1993. Each box trap was baited with two quail, one of which was injected with rubidium and the other with cesium (Anderson et al. 1990). (All experiments which involve the use of animals conform to guidelines contained in the Guide to the Care and Use of Experimental Animals, Vol. 1., Canadian Council on Animal Care and experimental protocol #C-91-46 has been approved by the University of Manitoba Animal Care Committee.) Rubidium and cesium can be used as host-blood markers to permit identification of hosts of mosquito blood meals and for detection of multiple feeding (Anderson et al. 1990). Two hosts were caged together, instead of singly, to determine if mosquitoes imbibe blood at equal frequencies from equally available sources. Additionally, the two host flock provided the opportunity for mosquitoes interrupted during feeding to resume, possibly on a second individual. With this experimental design, we were able to relate the frequency of multiple feeding to the joint probabilities of each host being fed on. Japanese quail (Coturnix japonica Temminck and Schlegel) were used in Manitoba and Northern Bob-white (Colinus virginianus L.) were used in Florida.
All mosquitoes collected in the box traps were identified, counted and sorted for engorged specimens. The size of blood meals was graded as £ ½ full or replete according to the criteria of Edman et al. (1975), who found that Culex nigripalpus Theobald with a partial blood meal £ ½ full were likely to continue blood feeding. In our study, mosquitoes with £ ½ of a blood meal and only one marker were assumed to have been interrupted at least once by the correspondingly-marked host before satiation (Edman et al. 1975). Multiple blood meals, or those with both markers were also assumed to have been interrupted and then resumed on the other host.
Blood-fed mosquitoes were analyzed individually for rubidium and cesium. A mosquito with only 1 marker was defined as having 1 blood meal. A mosquito with both markers was defined as having 2 blood meals. The probability of each quail being fed upon was calculated as the number of marked meals of one type divided by the total, marked meals of both types for each trap night. For the purposes of this calculation, each component of a multiple feeding is considered to be one meal. If there was no difference in tolerance to mosquito attack between the birds in a trap, we would expect that approximately equal numbers of blood meals during most trap-nights would be from each bird. The proportions of blood meals taken from each of the two possible hosts in each cage were plotted to illustrate the frequency with which blood feeding was distributed evenly or unevenly among the quail. For each sample size represented by the number of blood meals per trap night, confidence limits were calculated for the value 0.5 and for the proportion of blood meals that represented the split between rubidium-marked meals and cesium-marked meals. The difference between these proportion was considered significant if there was no overlap of the confidence intervals at p = 0.05 (2-tailed).
Only trap nights with at least 17 marked, engorged mosquitoes are presented, and estimates of the range in frequency of multiple feeding were based on these samples. According to the binomial expansion, 17 is the minimum number of blood-fed mosquitoes per sample for which an increase of one multiple blood meal does not result in rejection of the null hypothesis that the true frequency of multiple feeding is 5%. We initially estimated the overall frequency of multiple feeding by Cx. tarsalis, Cx. restuans and Cx. nigripalpus to be 5% based on combining the data for each species collection (Anderson and Brust, in press). The trap nights are sorted by magnitude of the proportion value that represents the split between the blood meals from the rubidium-injected quail and the cesium-injected quail. For the purposes of this paper, Cx. restuans and Cx. tarsalis collected in the same trap night in 1993 were combined as a single category, Culex. Both species are aviophilic and preliminary analysis indicated that feeding success of both species was approximately equal.
An index of interrupted feeding for the mosquitoes fed from each quail was calculated in the following way. For meals £ ½ full and only one marker, a probability of interruption of 1 was assigned to the correspondingly marked host for each meal. For multiple meals, a probability of interruption of 0.5 was assigned to each host for that particular blood meal (Burkot et al. 1988). The number of meals interrupted by a particular host equals the number of correspondingly-marked meals £ ½ full plus 0.5 X the number of multiple meals. The total number of blood meals originating on each quail equals the number of completely or partially fed female mosquitoes plus 0.5 X the number of multiple meals. The number of interrupted meals from one quail divided by the total number of blood meals originating on that quail is used as an index of the probability of interruption by that host. It is not an exact estimate because of cryptic meals. The indices of interruption for both quail in each pair were compared as a ratio of the greater to lesser probability of interruption for each trap night. The frequency distribution of this ratio was used to calculate the probability with which differences in the rate of interruption would occur for a given pair of quail. Only trap nights with at least 10 engorged mosquitoes from each quail were used for this analysis so that a difference in the proportion of interrupted meals due to 1 blood fed mosquito would not exceed 0.1. We examined the relationship between the probability of being fed on and the probability of interruption for each quail using regression analysis.
Burkot et al. (1988) showed mathematically that the highest frequencies of multiple feeding should occur in situations in which there is little or no difference in the probability of each of two hosts being fed upon. For a 2-host flock as in our experimental design, our null hypothesis is that the probability of feeding on each quail is 0.5. Therefore, as the proportion of blood meals from one bird deviates away from 0.5, we expect to see a corresponding decrease in the proportion of multiple meals. Accordingly, we examined the relationship between the probability of detecting multiple meals and the deviation from an even distribution of blood meals on each bird (0.5 rubidium and 0.5 cesium).
A total of 70 box trap collections (trap nights) had at least 17 blood fed Culex in each (Anderson and Brust, in press). In 25 of these trap nights from the Delta Marsh site in 1991, Cx. tarsalis was the dominant species (> 80% of mosquitoes), both in terms of total number of mosquitoes and in terms of number of blood-fed mosquitoes. In 20 trap night collections with at least 17 blood fed mosquitoes from Vero Beach, Cx. nigripalpus comprised more than 95% of the total. In 25 trap nights collected at Winnipeg in 1993, Cx. tarsalis and Cx. restuans were represented in approximately equal proportions and combined, accounted for more than 90% of all mosquitoes.
For Cx. tarsalis collected in 1991, the proportion of blood meals taken from 1 of the 2 quail in each box trap ranged from 0.047 to 0.954 (Fig. 1A). In 9 of 25 samples, the distribution of blood meals was skewed significantly away from 0.5 on each of the 2 birds (Fig. 1B). Not all of the apparently skewed proportions deviated significantly from 0.5 because confidence limits of proportions increase with decreasing numbers of individuals counted (Fig. 1A). For Cx. nigripalpus collected in 1992, the proportion of blood meals taken from 1 of the 2 quail in each box trap ranged from 0.154 to 0.778 (Fig. 1B). The distribution of blood meals was skewed significantly from 0.5 on each bird in only 1 of 20 samples, partly because of the low numbers of mosquitoes per trap night (Fig. 1B). For Cx. tarsalis/Cx. restuans combined collections from 1993, the proportion of blood meals taken from 1 of the 2 quail in each box trap ranged from 0 to 1.0 (Fig. 1C). The distribution of blood meals was skewed significantly from 0.5 on each bird in 19 of 25 samples (Fig. 1C). The density of host-seeking Cx. tarsalis in 1991 and Cx. tarsalis/Cx. restuans in 1993 was higher than for Cx. nigripalpus collected in 1992 (Fig. 1).
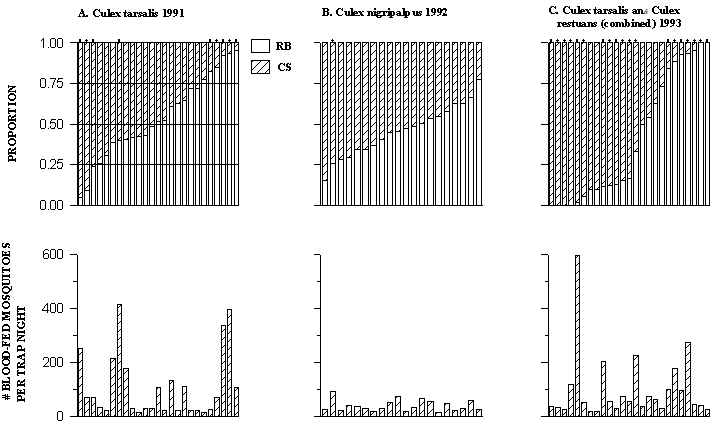
Figure 1. Distribution of mosquito blood feeding on each of two
quail for each trap night with at least 17 blood fed, marked Culex
mosquitoes. Each bar represents 1 trap night. Black bars
represent the proportion of blood meals marked with rubidium. White
bars represent the proportion of blood meals marked with cesium.
Bars capped by filled circles denote proportions of blood meals
significantly different from 0.5 on each bird. The number of
marked mosquitoes for each trap night is given by the height of
the crosshatched bar in the bottom graph. A) Cx. tarsalis
collected in 1991 (25 trap nights), B) Cx. nigripalpus collected
in 1992 (20 trap nights), C) Cx. tarsalis and Cx.
restuans combined, collected in 1993 (25 trap nights)
A total of 40 trap night collections contained at least 10 blood fed mosquitoes marked with rubidium and 10 marked with cesium. For 15 trap night collections of Cx. tarsalis from 1991, the proportion of interrupted blood meals ranged from 0.032 to 0.583 for the rubidium-marked quail and from 0.053 to 0.727 for the cesium-marked quail (Fig. 2A). In 8 of 15 cases, interrupted blood meals were from 2 to 8.8 times more likely from 1 bird relative to the other in a given pair (Fig. 2A). For 14 trap night collections of Cx. nigripalpus from 1992, the proportion of interrupted blood meals ranged from 0.017 to 0.571 for the rubidium-marked quail and from 0.119 to 0.607 for the cesium-marked quail (Fig. 2B). In 5 of 14 cases, interrupted blood meals were from 2 to 6.9 times more likely from 1 bird relative to the other in a given pair (Fig. 2B). For 11 trap night collections of Cx. tarsalis/Cx. restuans from 1992, the proportion of interrupted blood meals ranged from 0.056 to 0.95 for the rubidium-marked quail and from 0.138 to 0.548 for the cesium-marked quail (Fig. 2C). In 5 of 11 cases, interrupted blood meals were from 2 to 4.7 times more likely from 1 bird relative to the other in a given pair (Fig. 2C).
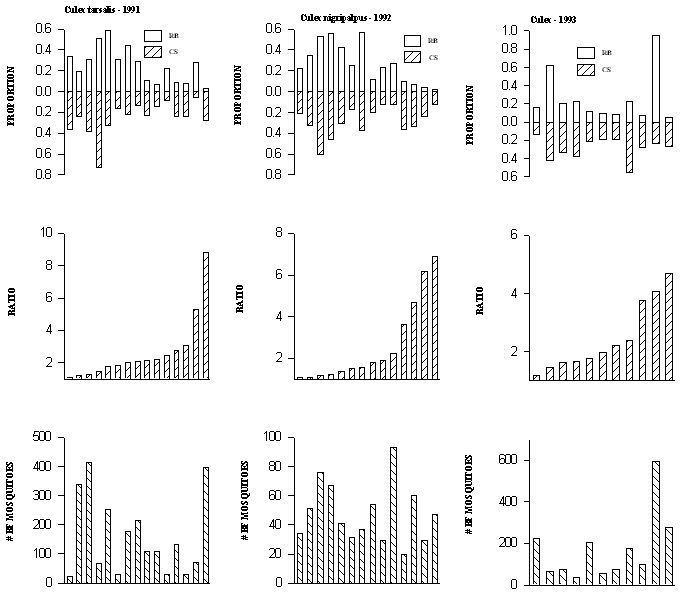
Figure 2. Proportions of interrupted blood meals (see text for
definition) on each of rubidium and cesium marked quail for each
trap night (top graphs). The middle graph contains the ratio of
the larger proportion to the smaller from the top graph. The
number of marked mosquitoes for each trap night is given by the height
of the crosshatched bar in the bottom graph. Each bar represents
a trap night. There are at least 10 rubidium-marked mosquitoes
and 10 cesium-marked mosquitoes for each trap night represented.
A) Cx. tarsalis collected in 1991 (15 trap nights), B) Cx.
nigripalpus collected in 1992 (14 trap nights), C) Cx. tarsalis
and Cx. restuans combined, collected in 1993 (11 trap
nights)
The relationship between the probability that meals from a given bird would be £ ½ full and the probability of the same bird being fed upon is shown in Figure 3. The proportion of interrupted meals from a given quail was negatively correlated with the proportion of blood feeding on that quail (p = 0.0026) (Fig. 3). The proportion of detectable multiple meals was negatively correlated with the degree of skewness away from equal blood feeding success on each quail (p < 0.0001) (Fig.4).
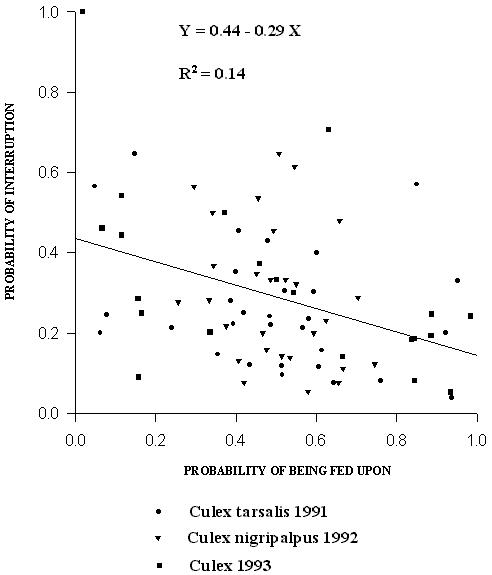
Figure 3. Relationship between the probability of a blood meal £ ½ full and the
probability of being fed upon for each quail from trap nights
with at least 10 each of rubidium-marked mosquitoes and
cesium-marked mosquitoes. Symbol shapes denote the year of
collection and species of Culex as in the figure legend. Regression
is significant (p = 0.0026).
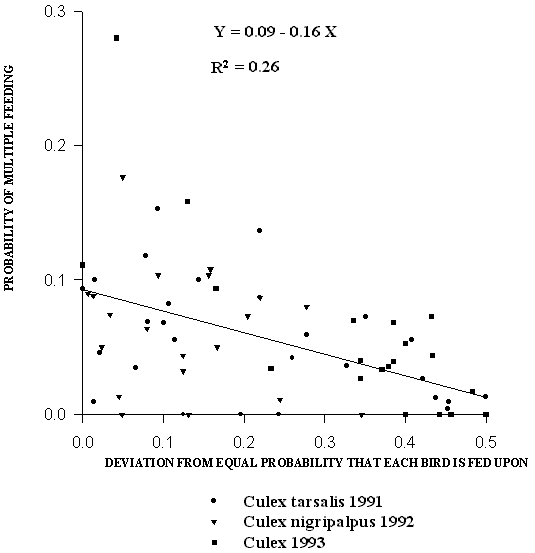
Figure 4. Relationship between probability of multiple feeding
and the skewness away from equal probability of mosquitoes
feeding on each bird. Each trap night has at least 17 blood-fed,
marked mosquitoes. Regression is significant (p < 0.0001)
Individual quail of the same species, size, sex and age vary considerably in the degree to which mosquitoes ingest blood from them (Fig. 1). The degree of skew in the distribution of blood meals between 2 possible hosts was greater for Cx. tarsalis collected in 1991 and for Cx. tarsalis/Cx. restuans collected in 1993 than for Cx. nigripalpus collected in 1992 (Fig. 1). This was likely due to the lower number of blood-fed mosquitoes per pair of birds in 1992 compared with the number of mosquitoes in 1993 (Fig. 1). Confidence limits of proportions increase as the number of individuals counted declines. Thus it is more difficult to reject a null hypothesis that the proportion of blood meals from each bird is equal to 0.5 for trap nights with small numbers of blood fed mosquitoes.
The proportion of mosquitoes with interrupted blood meals varied from 0.01 to 0.95 for individual quail in our study (Fig. 2). These estimates are based on physical criteria associated with blood meal size. It is difficult to compare them directly with estimates of interrupted feeding derived from the equation of Burkot et al. (1988) as used by Scott et al. (1993) for a different species of mosquito. However, in our study and in that of Scott et al. (1993), the estimates of the probability of interruption for blood-feeding mosquitoes varied from close to 0 to 1.0.
The often skewed patterns of blood feeding observed in our study were the result of differences in the intensity of defensive behavior exhibited by the individual hosts (Anderson and Brust, unpublished data). In other studies, feeding success of mosquitoes varied considerably from one individual to another of a particular host species (Dow et al. 1957, Kale et al. 1972), although Edman and Scott (1987) concluded that individual differences among hosts were not the most important factor that determined mosquito feeding success.
Interruption of blood feeding by quail is a trait which is negatively correlated with the probability that mosquitoes will obtain blood (Fig. 3). Our results are consistent with those of Edman et al. (1972), who showed that fewer mosquitoes overall, obtained blood and more took partial blood meals from the most defensive avian hosts. Although quail are not the most important avian hosts for wild populations of Cx. tarsalis, Cx. restuans and Cx. nigripalpus, variation among wild avian hosts exists (Dow et al. 1957, Kale et al. 1972) and is likely an important source of uncertainty and patchiness in the blood resource available to host seeking mosquitoes.
Variation in the degree to which individual hosts tolerate mosquito attack may be a significant factor that determines the average host contact rate of mosquitoes from one location to another because those interrupted during feeding may resume on other hosts (Anderson and Brust, in press). Serology may be used to measure multiple feeding by mosquitoes (Scott et al. 1993, Burkot et al. 1988), but it may not detect cryptic multiple meals. Boreham and Garrett-Jones (1973) recognized the potential importance of cryptic multiple feeding and developed a series of probabilities to account for the ways in which a mosquito might take one or two meals given that at least two types of hosts are available. They assumed that hosts are attacked randomly for each feeding attempt, although the probability of successful feeding is not necessarily equal among potential hosts and not necessarily equal even if both hosts are available during both blood feeding attempts.
Burkot et al. (1988) recognized that, to calculate the frequency of cryptic multiple feeding by the method of Boreham and Garrett-Jones (1973), and in the absence of specific information about the tendency of different hosts to interrupt blood feeding mosquitoes, one must assume that the probability of interruption on different hosts is equal. Burkot et al. (1988) used this implicit assumption to calculate the overall probability of interruption for three species of Anopheles in Papua New Guinea based on the observed proportion of patent, multiple meals and the probability of each host being fed on. The method of Burkot et al. (1988) was used by Scott et al. (1993) to calculate the probability of interruption for Aedes aegypti L. from serological data on host selection and patent multiple feeding. However, no data were presented in either study to confirm the assumption that different hosts interrupt blood feeding mosquitoes at the same frequency, even though the probabilities of each type of host being fed on varied considerably from location to location. In both of these studies, more than one type of host as well as more than one individual host of each type were involved with the attendant potential for variation among hosts operating at 2 levels. Based on our data, it is not valid to assume that individual hosts interrupt blood feeding mosquitoes at the same rate, especially when the probability of hosts being fed on varies (Fig. 3). In 18 of 40 cases in which we calculated the relative rate of interrupted blood feeding, 1 quail was from 2 to 8 times more likely to interrupt mosquitoes than was the other immediately available quail (Fig. 2). Additionally, Burkot et al. (1988) demonstrated graphically that the proportion of cryptic mixed feeding is significantly altered in situations in which the probability of interruption differs among potential hosts. Thus, calculations are suspect that depend on assuming homogeneity among hosts with respect to tendency to interrupt blood feeding mosquitoes.
The equation used by Burkot et al. (1988) provides an indirect means of estimating total interrupted feeding if the proportion of patent multiple meals is known. However, several authors (Scott et al. 1993; Burkot 1988; Burkot et al. 1988) considered such estimates to be equivalent to the probability of multiple feeding, even though the total proportion of feeding attempts that are interrupted during one feeding are actually estimated. Some, but not all interrupted feeds are completed on a second host and only this smaller proportion represents multiple host contacts. Some mosquitoes interrupted before satiation may not continue to blood feed or they may not successfully imbibe more blood during subsequent attempts. Thus, the value, ‘I’, estimated according to the method of Burkot et al. (1988) can not be considered an estimate of multiple host contacts.
We have shown that a significant amount of variation in observed frequencies of multiple feeding is correlated with relative differences in the degree to which individual quail are fed upon (Fig. 4). Burkot et al. (1988) calculated that patent multiple feeding is expected to be maximal when the probability of feeding on either of 2 hosts is equal (0.5). This calculation rests on the assumption that, for each feeding attempt, mosquitoes attack hosts of the same type at random with respect to their physical availability, but successfully blood feed depending on the tolerance of the host. Any given meal is most likely to come from the host most tolerant to attack. Consequently, if one host is very intolerant relative to the other, few mosquitoes will imbibe any blood from the intolerant host and most partial blood meals will have been interrupted and resumed on the same host. Alternatively, if both hosts are relatively equal in tolerance to mosquito attack, an interrupted meal is equally likely to be resumed on either of the two hosts. The total amount of multiple feeding would depend on the tendency of both hosts to interrupt blood feeding attempts. Thus, the proportion of patent multiple meals would be highest for situations in which many meals on both hosts are interrupted and the two hosts are very similar in terms of the probability of being fed on. Our data are consistent with this model (Fig. 4). One implication of this is that the host contact rate of mosquitoes may increase substantially in habitats in which the most available hosts are uniformly intolerant of mosquito attack.
Multiple feeding may occur by two fundamentally different mechanisms. Ae. aegypti (Scott et al. 1993) and many Anopheles (Klowden and Briegel 1994) may be gonotrophically discordant and thus blood feed several times between egg batches to supplement nutritional reserves. Alternatively, gonotrophically concordant species may contact more than one host because an earlier feed was interrupted before satiation (Klowden 1988), usually by host defensive behavior (Davies 1991). In the latter situation, serial feeding attempts are likely to occur during a short period of time such as a single night. Refeeding avidity tends to decrease as the delay between serial meals is increased (Edman et al. 1975). Our results with respect to interrupted and multiple feeding by Cx. tarsalis, Cx. restuans and Cx. nigripalpus are most consistent with the situation outlined for gonotrophically concordant species. In either situation, even relatively low frequencies of multiple host contacts may be important because of additional opportunities for the mosquito to acquire or transmit pathogens (Smith 1987).
We thank Terry Galloway (University of Manitoba) for helpful comments on the manuscript. We thank the staff of the University of Manitoba Field Station and the Florida Medical Entomology Laboratory for logistical and material support and the Freshwater Institute for use of a flame spectrophotometer. We gratefully acknowledge financial assistance in the form of a University of Manitoba Graduate Fellowship and an Entomological Society of Canada research travel grant to RAA. We also acknowledge operating grants from the Canadian Shield Foundation and the Natural Sciences and Engineering Research Council of Canada to RAB.
Anderson, R. A. and Brust, R. A. In press. Field evidence for multiple host contacts during blood feeding by Culex tarsalis, Culex restuans and Culex nigripalpus (Diptera: Culicidae). J. Med. Entomol.
Anderson, R. A., Edman, J. D. and Scott, T. W. 1990. Rubidium and cesium as host blood-markers to study multiple blood feeding by mosquitoes (Diptera: Culicidae). J. Med. Entomol. 27: 999-1001.
Boreham, P. F. L. and Garrett-Jones, C. 1973. Prevalence of mixed blood meals and double feeding in a malaria vector (Anopheles sacharovi Favre). Bull. Wld. Hlth. Org. 48: 605-614.
Burkot, T. R. 1988. Non-random host selection by Anopheline mosquitoes. Parasitol. Today 4: 156-162.
Burkot, T. R., Graves, P. M., Paru, R. and Lagog, M. 1988. Mixed blood feeding by the malaria vectors in the Anopheles punctulatus complex (Diptera: Culicidae). J. Med. Entomol. 25: 205-213.
Davies, C. R. 1990. Interrupted feeding of blood-sucking insects: causes and effects. Parasitol. Today 6: 19-22.
de Moor, P. P. and Steffens, F. E. 1970. A computer-simulation model of an arthropod-borne virus transmission cycle, with special reference to chikingunya virus. Trans. Royal Soc. Trop. Med. Hyg. 64: 927-934.
Dye, C. 1992. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 37: 1-19.
Dow, R. P., Reeves, W. C. and Bellamy, R. E. 1957. Field tests of avian host preference of Culex tarsalis Coq. Am. J. Trop. Med. Hyg. 6: 294-303.
Edman, J. D. and Downe, A. E. R. 1964. Host-blood sources and multiple-feeding habits of mosquitoes in Kansas. Mosquito News. 24: 155-160.
Edman, J. D. and Scott, T. W. 1987. Host defensive behaviour and the feeding success of mosquitoes. Insect Sci. Applic. 8: 617-622.
Edman, J. D., Cody, E. and Lynn, H. 1975. Blood-feeding activity of partially engorged Culex nigripalpus (Diptera: Culicidae). Entomol. Exp. Applic. 18: 261-268.
Edman, J. D., Webber, L. A. and Kale, H. W. II. 1972. Effect of mosquito density on the interrelationship of host behavior and mosquito feeding success. Am. J. Trop. Med. Hyg. 21: 487-491.
Kale, H. W., Edman, J. D. and Webber, L. A. 1972. Effect of behavior and age of individual ciconiiform birds on mosquito feeding success. Mosquito News 32: 343-350.
Klowden, M. J. 1988. Factors influencing multiple host contacts by mosquitoes during a single gonotrophic cycle, pp. 29-36. In: Scott, T. W. and Grumstrup-Scott, J., [eds]. The role of vector-host interactions in disease transmission. Misc. publ. no. Entomol. Soc. Am. 68
Klowden, M. J. and Briegel, H. 1994. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between Anopheles and Aedes (Diptera: Culicidae). J. Med. Entomol. 31: 618-622.
Scott, T. W., Chow, E., Strickman, D., Kittayapong, P., Wirtz, R. A., Lorenz, L. H. and Edman, J. D. 1993. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J. Med. Entomol. 30: 922-927.
Smith, C. E. G. 1987. Factors influencing the transmission of western equine encephalomyelitis virus between its vertebrate maintenance hosts and from them to humans. Am. J. Trop. Med. Hyg. (Suppl.) 37: 33s-39s.
Webber, L. A. and Edman, J. D. 1972. Anti-mosquito behaviour of ciconiiform birds. Animal Behav. 20: 228-232.