Robert A. Anderson and Reinhart A. Brust
Department of Entomology, University of Manitoba
Winnipeg, Manitoba, Canada R3T 2N2
Some mosquitoes attracted to bait hosts have ingested blood recently (Mitchell and Millian 1981; Trpis and Hausermann 1986). Though blood meal identification studies repeatedly have shown that many North American Culex vectors of encephalitis viruses ingest blood from more than one type of animal in a single gonotrophic cycle (Edman and Downe 1964; Cupp and Stokes 1976), no information is available with regard to multiple feeding by most species of Culex on conspecific avian hosts.
We use the term multiple feeding to describe the situation in which a mosquito ingests some blood from at least 2 hosts during a single gonotrophic cycle. This is distinct from the situation in which a mosquito is interrupted during blood uptake, but returns to the same host to complete the blood meal. Multiple feeding during a single gonotrophic cycle may occur for either of 2 distinct reasons. In 1 case, 2 or more hosts may be bitten if mosquitoes are prevented from acquiring sufficient blood from one host to induce neural and hormonal mechanisms which inhibit further blood feeding (Klowden 1988). Interruption of blood uptake before satiation is associated most commonly with defensive behavior by the hosts (Edman and Scott 1987, Davies 1990). This would be the prevalent situation for gonotrophically concordant species that require only 1 blood meal per reproductive cycle. Alternatively, species that require several blood meals for oogenesis or for metabolic reserves may continue to host seek (perhaps daily) between one oviposition event and the next. This is the case for some Anopheles (Klowden and Briegel 1994) and Aedes aegypti (Trpis and Hausermann 1986; Scott et al. 1993).
The objective of our study was to use a novel marking technique to determine if Culex tarsalis Coquillett, Culex restuans Theobald and Culex nigripalpus Theobald take multiple meals on conspecific avian hosts. Such information would provide insight as to whether multiple feeding on conspecific hosts is a behavioral phenomenon that has been overlooked in previous blood feeding studies based on serological methodology.
Following the preliminary work of Kimsey and Kimsey (1984), in which rubidium was used as a host-blood marker, Anderson et al. (1990) developed a blood-marking technique in which rubidium is injected into 1 of 2 hosts (such as quail) and cesium is injected into the other. Pairs of birds marked in this way are made available to host-seeking mosquitoes and the blood meals are assayed for the presence of both rubidium and cesium. This technique permits the identification of mosquitoes that have obtained blood from 1 or both birds in the pair, although interrupted meals resumed on the same host are not detectable.
Blood feeding by wild mosquitoes was studied in Manitoba, Canada, at Delta Marsh during 1991 and at Winnipeg during 1993. Delta Marsh is a large freshwater marsh (>20,000 ha) at the south end of Lake Manitoba. The Winnipeg site is located on the University of Manitoba campus along the Red River. Both sites provide extensive breeding habitat for passerine birds and mosquitoes such that large populations of both coincide during the summer.
Box traps (30 by 30 by 30 cm) (Fig. 1) with baffled, slotted entrances (narrowing from 30 by 8 cm to 30 by 2 cm) on the underside were used to capture mosquitoes attracted to the quail. The baffled entrances were constructed of fine mesh to permit downward movement of host odors. Traps were suspended » 1 m above the ground on the edge of wooded areas at each location in Manitoba. Traps were baited with pairs of numbered, Japanese quail (Coturnix japonica Temminck & Schlegel). Quail were 8-12 wks old and weighed, on average, 120 g. Overall, 102 pair of quail were used during 1991, and 40 pair of quail were used during 1993.
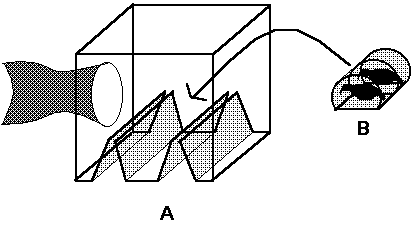
Figure 1. Box trap used to collect blood-fed mosquitoes attracted
to marked quail. (A) Trap design. (B) Wire cage for quail. Arrow
indicates the position of the wire cage within the trap
Quail were placed in cylindrical, wire cages (25 by 15 cm diameter, mesh size 1.3 by 1.3 cm) (Fig. 1) which were inserted through the stocking sleeve and placed between the baffles in the box traps (Fig. 1). (All experiments that involved the use of animals conformed to guidelines contained in the "Guide to the Care and Use of Experimental Animals", vol. 1., Canadian Council on Animal Care, and experimental protocol #C-91-46 was approved by the University of Manitoba Animal Care Committee.) These small cages restricted the quail from access to the inside surfaces of the box trap to prevent resting mosquitoes from being eaten; however, the quail had sufficient room to turn around, stretch and, groom themselves. One quail of each pair was injected with the alkali metal rubidium and the other with cesium. Rubidium and cesium circulate in the blood for several days, are assayed easily in blood fed mosquitoes, and permit the unequivocal determination of which bird was fed upon by each mosquito in a particular trap (Anderson et al. 1990). If both metals are present in a single mosquito, then that mosquito obtained blood from both hosts during the period of exposure.
Box traps were placed in their field locations » 30 min before sunset and were collected within 30 min of sunrise. At collection, the no-return entrances were sealed with foam rubber plugs and the quail cages were removed through a sleeve of surgical stocking. Quail were returned to the flock cage and box traps were placed in a freezer at -20°C to kill the mosquitoes.
The same method was used to collect blood-fed Cx. nigripalpus in Florida in August of 1992, except that Northern Bob-white (Colinus virginianus L.) were used as bait animals to attract Cx. nigripalpus and to measure multiple feeding by this species. The average weight of the northern bob-white was 95 g. Twenty-six pairs of quail were used during this part of the study. All mosquitoes were collected in the hardwood hammock that surrounds the Florida Medical Entomology Laboratory at Vero Beach. Box traps were of similar design to those used in Manitoba, except that they were made from clear acrylic (plastic) rather than plywood. Exposure times also were from before sunset to after sunrise, but the number of hours exposure was not equivalent because of significant latitudinal differences between central Florida and Manitoba.
Mosquitoes collected from all quail-baited traps were identified and blood-fed individuals were retained for rubidium and cesium analysis by atomic emission flame spectrophotometry (Anderson et al. 1990). The mosquitoes collected in one box trap during a given sunset to sunrise collection period were defined as a sample. Thus, each sample provided a replicate measure of the frequency (expressed as percent) of multiple host contacts. Only blood-fed mosquitoes positive for either rubidium or cesium were included in the number blood fed per sample. The few blood-fed mosquitoes in some of the samples that were negative for both rubidium and cesium presumably obtained blood from other sources and were excluded from the calculation described above.
The frequency of multiple feeding per sample was calculated as the number of blood-fed mosquitoes with both rubidium and cesium, divided by the total, marked, blood-fed individuals of that species. Initially, all blood-fed Cx. tarsalis collected in 1991 were lumped together to calculate the overall frequency of multiple feeding by this species. The same approach was used for Cx. nigripalpus collected in 1992 and for Cx. restuans and Cx. tarsalis collected in 1993. Estimates of the range in frequency of multiple feeding were based on samples in which at least 17 mosquitoes blood fed on the quail. According to the binomial expansion, 17 is the minimum number of blood-fed mosquitoes per sample for which an increase of 1 multiple blood meal does not result in rejection of the null hypothesis that the true frequency of multiple feeding is 5%. In other words, samples smaller than 17 were considered unreliable. We initially estimated the overall frequency of multiple feeding by Cx. tarsalis, Cx. restuans and Cx. nigripalpus to be 5% based on combining the data for each species collection. The mean, standard error and confidence limits of the frequency of multiple feeding by species and year of collection were calculated directly from the quotients of number of 2-host meals divided by the number of quail-fed mosquitoes multiplied by 100.
In addition to the direct evidence of multiple feeding by double-marked mosquitoes described above, 2 sources of indirect evidence for other mosquitoes with high potential for refeeding are provided. First, blood meals were assigned to 1 of 4 size classes: trace, one-quarter full, one-half full, and full according to the criteria of Edman et al. (1975) to provide information on the extent of multiple feeding by partially and fully engorged mosquitoes. Edman et al. (1975) found that Cx. nigripalpus with half a full blood meal or less were more likely to refeed than were females with greater than a half full blood meal. In our study, mosquitoes with a half blood meal or less (partial meals) and both rubidium and cesium were individuals that had taken blood from 2 hosts and were considered likely to refeed again. The means of the percentages of multiple feeding for each species collection were compared by ANOVA (Statistix 1992). Confidence limits of the percentage of multiple feeding that resulted in partial blood meals were calculated from the binomial expansion (Sokal and Rohlf 1981).
Second, mosquitoes in the box traps that contained fresh blood, but negative for both markers were assumed to be host-seeking, although already engorged. Very few (<<1% of all mosquitoes collected in the box traps) were gravid or teneral females or males.
In Manitoba in 1991, 13,857 female mosquitoes were collected in 165 trap-nights with pairs of marked quail as bait. Of these mosquitoes, 5,218 were Cx. tarsalis and 3,102 (59%) had ingested blood from at least 1 quail. In Manitoba in 1993, 4,141 female mosquitoes were collected in 40 trap-nights of which 2,027 were Cx. restuans and 1,764 were Cx. tarsalis. Overall, 1,409 (70%) of the Cx. restuans and 1,207 (68%) of the Cx. tarsalis ingested blood from at least 1 quail. In Florida in 1992, 2,110 female mosquitoes were collected, of which 2,041 (97%) were Cx. nigripalpus; 857 (42%) had ingested blood from at least 1 of the quail.
Overall, 331 of 6,575 (5.03%) engorged Culex took blood from 2 quail. The percentage and sample size of multiple host contacts by species were 5.09% of 3,102 engorged Cx. tarsalis collected in 1991, 4.14% of 1,207 engorged Cx. tarsalis collected in 1993, 5.39% of 1,409 engorged Cx. restuans collected in 1993, and 5.48% of 857 engorged Cx. nigripalpus collected in 1992. The range in frequency of patent multiple blood meals is given for samples with at least 17 marked, blood-fed mosquitoes in Table 1. The frequency of multiple blood feeding did not differ among the species studied.
| Table 1. Variation in the frequency of multiple host contacts by Culex tarsalis and Culex restuans on Japanese quail and Culex nigripalpus on northern bob-white. There are no significant differences among the mean percentages (ANOVA, Analytical Software 1992). LCL, lower confidence limit; UCL, upper confidence limit; confidence interval = 95%. n, number of samples in which > 17 mosquitoes blood-fed on >1 quail. | |||||
% Frequency |
|||||
| Species |
|
Mean±SE |
LCL-UCL |
Min-Max |
n |
Culex tarsalis |
1991 |
5.5 ± 0.88 |
3.7 - 7.3 |
0 - 15.2 |
25 |
|
1993 |
5.0 ± 1.34 |
2.2 - 7.8 |
0 - 18.5 |
20 |
Culex restuans |
|
5.5 ± 2.00 |
1.4 - 9.6 |
0 - 33.3 |
18 |
Culex nigripalpus |
|
6.2 ± 1.02 |
4.1 - 8.4 |
0 - 17.6 |
20 |
The number and frequency (expressed as percent) of mosquitoes that took blood from both quail, but for which the blood meals were graded as partial are given by species in Table 2. Multiple host contacts that resulted in partial meals by Cx. nigripalpus occurred at a significantly greater frequency than for Cx. tarsalis collected in 1991.
Table 2. Number and frequency of multiple blood meals that were partial (those for which the total volume was < ½). Percentages followed by the same letter are not significantly different (Sokal and Rohlf 1981). |
||||
Species |
|
Partial/Multiple |
% |
LCL-UCL |
Culex tarsalis |
1991 |
21/158 |
13.3a |
9.0 - 19.6 |
|
1993 |
8/50 |
16.0ab |
8.6 - 29.1 |
Culex restuans |
|
13/76 |
17.1ab |
10.4 - 27.5 |
Culex nigripalpus |
|
15/47 |
31.9b |
20.9 - 47.1 |
The number and frequency of blood-fed mosquitoes that had ingested blood before attraction to the quail (unmarked with either rubidium or cesium) are given in Table 3. These are minimum estimates, because previously engorged mosquitoes that obtained blood from either quail would not be included in this category. The frequency of unmarked blood meals was greatest for Cx. nigripalpus and the 1993 Cx. tarsalis collection. More than 85% of unmarked blood meals were partial according to the grading scheme of Edman et al. (1975) (Table 3).
Table 3. Number of blood-fed mosquitoes that acquired blood (unmarked with rubidium or cesium) before entering traps baited with quail. Numbers followed by the same letters are not significantly different (Sokal and Rohlf 1981). aTotal blood-fed mosquitoes in which neither rubidium nor cesium was detected: defined as unmarked. bMarked + unmarked, blood-fed mosquitoes in quail baited traps. cUnmarked blood meals as a percentage of all blood-fed mosquitoes. dPercentage of unmarked, partial blood meals, according to the criteria of Edman et al. (1975). All percentages in this column are not significantly different (Sokal and Rohlf 1981). |
|||||
Species |
|
Totala/Blood-fedb |
%c |
Partial/Totala |
%d |
Culex tarsalis |
1991 |
39/3141 |
1.2a |
38/39 |
97.4 |
|
1993 |
25/1232 |
2.0ab |
24/25 |
96.0 |
Culex restuans |
|
15/1424 |
1.0a |
13/15 |
86.7 |
Culex nigripalpus |
|
31/ 888 |
3.5b |
27/31 |
87.1 |
Blood from 2 or more hosts, distinguishable at the species or family level by serology, has been demonstrated many times in the guts of individual mosquitoes of many species (Edman and Downe 1964; Cupp and Stokes 1976). However, multiple feeding on individuals of the same species (cryptic meals) has been demonstrated only for a few anopheline and culicine species feeding on humans with distinct ABO blood groups or haptoglobins (Boreham et al. 1978; Boreham and Lenahan 1979; Burkot et al. 1988). Avians are important hosts for Cx. tarsalis, Cx. restuans, and Cx. nigripalpus (Washino and Tempelis 1983). Often, a few species of passerine birds are most important for virus amplification (Holden et al. 1973). Traditional serological methods are not adequate for detection of multiple feeding on conspecific hosts, but multiple feeding in this situation may be of importance in the enzootic transmission of virus.
Furthermore, many avian species often aggregate in colonial nesting areas, on the nest, at roosts, and at feeding sites (Weatherhead 1981, 1983). Behavioral studies of interactions between host-seeking mosquitoes and avian hosts have shown that birds may interrupt blood feeding such that mosquitoes potentially may contact more than one host of the same species in the course of obtaining a full blood meal (Kale et al. 1972; Webber and Edman 1972).
In our study, Cx. tarsalis, Cx. restuans, and Cx. nigripalpus took multiple meals from conspecific avian hosts. Although the overall frequencies were close to 5%, the maximum observed frequencies ranged from 13.6% for Cx. tarsalis to 33.3% for Cx. restuans. Edman (1974) recorded <1% multiple feeding by Cx. nigripalpus from Florida. Edman and Downe (1964) recorded overall percentages of multiple meals by 13 species of mosquitoes in 5 genera, including Cx. tarsalis, (21.5% multiple), Cx. salinarius Coquillett (36.7% multiple), and Cx. pipiens L.(20% multiple). Cupp and Stokes (1976) noted that 13% of 328 Cx. salinarius took multiple meals. Anderson et al. (1990) observed that 19% of Cx. quinquefasciatus Say ingested blood from 2 chickens in the laboratory. Additionally, multiple feeding by Culex mosquitoes on conspecific hosts is not restricted to ornithophagic species. For example, Boreham et al. (1978) found that from 7.5% to 19.8% of Cx. quinquefasciatus Say collected in Kisumu, Kenya, imbibed blood from 2 or more human hosts.
The box trap used in our study was designed to retain mosquitoes during and after blood feeding on the quail, and this may have resulted in unnaturally high multiple feeding by keeping the mosquitoes in close proximity to hosts. Also, our use of quail as model avian hosts may not reflect perfectly the response of mosquitoes to passerine birds. However, with one exception, the estimates of the frequency of multiple feeding on conspecific hosts by the species in our study accord well with estimates from other studies of multiple feeding by Culex mosquitoes on natural hosts (Edman and Downe 1964; Cupp and Stokes 1976).
Despite the potential bias presented by the trap design, we feel that the frequencies of multiple feeding observed in our study likely represent an underestimate of the frequency of host contacts that involve secretion of saliva. We measured only host contact based on blood uptake. Mosquitoes may salivate into a host without ingesting blood (Ribeiro 1987). Furthermore, many mosquitoes (up to 31.9% in our study, Table 2) that had made at least 2 host contacts and ingested detectable amounts of blood were likely to blood feed again because the total amount of blood obtained from 2 hosts probably was still not sufficient to inhibit further blood feeding (Edman et al. 1975). Additionally, we observed that up to 3.5% of blood-fed mosquitoes attracted to the quail had first ingested blood from other sources (Table 3).
Our data provide a basis for challenging the assumption of 1 host contact per mosquito per gonotrophic cycle for the purposes of modeling vectorial capacity (Smith 1987). Multiple host contacts may increase the number of opportunities for individual mosquitoes to both acquire and transmit virus. Contact between mosquitoes and vertebrate hosts appears twice in the vectorial capacity model as multiplied terms. Effectively, disease transmission increases as the square of the increase in frequency with which mosquito vectors feed on amplifying vertebrate hosts (Dye 1992). For example, 5% multiple feeding may result in more than a 10% increase in transmission (Fig. 2). Vectorial capacity may be underestimated if it is assumed that each mosquito bites 1 host each gonotrophic cycle. Our contention that transmission may increase as a result of multiple feeding rests on 2 assumptions. First, we assume that small meals taken during multiple feeding by uninfected mosquitoes produce infective vectors. Second, we assume that a single mosquito is capable of delivering virus to more than one host during serial probing. Once infected with Western Equie Encephalitis Virus or Saint Louis Encephalitis Virus, Cx. tarsalis generally are infected for life (Henderson et al. 1979; Mitchell et al. 1980; Hardy 1987). Multiple feeding by 3 species of North American Culex occurred in 2 geographically distinct locations. Clearly, more attention should be paid to the dynamics of interrupted and multiple blood feeding on similar and dissimilar hosts with regard to disease transmission.
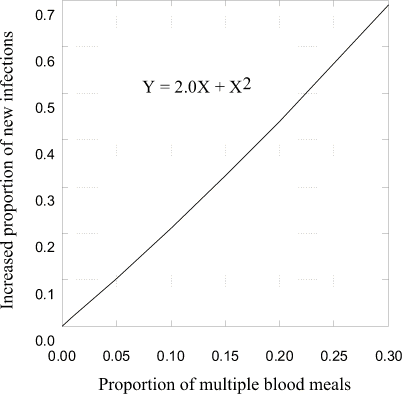
Figure 2. Relationship between increase
in reproductive rate (R) of an arbovirus during amplification in
an avian population and increase in vectorial capacity caused by
multiple feeding of mosquitoes. The relationship is calculated
for the range in multiple feeding (0-30%) observed in our study,
and according to the formula advanced by Smith (1987). Increase
in transmission is calculated relative to R = 1 for stable
tranmsission. From Smith’s model (1987), 2 parameters associated
with mosquito-host contact, "M" (average number mosquitoes
per host per day) and "B" (average number of blood
meals per mosquito per day), have been increased by the
proportion representative of multiple feeding such that the calculation
yields a squared relationship between the increase in host contacts
caused by multiple feeding and the increase in R.
We thank John Edman (University of Massachusetts) and Terry Galloway (University of Manitoba) for helpful comments on the manuscript. We thank the staff of the University of Manitoba Field Station and the Florida Medical Entomology Laboratory for logistical and material support, and the Fresh Water Institute for use of a flame spectrophotometer. We gratefully acknowledge financial assistance in the form of a University of Manitoba Graduate Fellowship and an Entomological Society of Canada research travel grant to R.A.A. We also acknowledge operating grants from the Canadian Shield Foundation and the Natural Sciences and Engineering Research Council of Canada to R.A.B.
Anderson, R. A., Edman, J. D. and Scott, T. W. 1990. Rubidium and cesium as host blood-markers to study multiple blood feeding by mosquitoes (Diptera: Culicidae). J. Med. Entomol. 27: 999-1001.
Boreham, P. F. L. and Lenahan, J. K. 1979. Studies on multiple feeding by Anopheles gambiae s.l. in a Sudan savanna area north of Nigeria. Trans. R. Soc. Trop. Med. Hyg. 73: 418-423.
Boreham, P. F. L., Chandler, J. A and Jolly, J. 1978. The incidence of mosquitoes feeding on mothers and babies at Kisumu, Kenya. J. Trop. Med. Hyg. 81: 63-67.
Burkot, T. R., Graves, P. M., Paru, R. and Lagog, M. 1988. Mixed blood feeding by the malaria vectors in the Anopheles punctulatus complex (Diptera: Culicidae). J. Med. Entomol. 25: 205-213.
Cupp, E. W. and Stokes, G. M. 1976. Feeding patterns of Culex salinarius Coquillett in Jefferson Parish, Louisiana. Mosq. News 36: 332-335.
Davies, C. R. 1990. Interrupted feeding of blood-sucking insects: causes and effects. Parasitol. Today 6: 19-22.
Dye, C. 1992. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 37: 1-19.
Edman, J. D. 1974. Host-feeding patterns of Florida mosquitoes III. Culex (Culex) and Culex (Neoculex). J. Med. Entomol. 2: 95-104.
Edman, J. D. and Downe, A. E. R. 1964. Host-blood sources and multiple-feeding habits of mosquitoes in Kansas. Mosq. News 24: 155-160.
Edman, J. D. and Scott, T. W. 1987. Host defensive behaviour and the feeding success of mosquitoes. Insect Sci. Applic. 8: 617-622.
Edman, J. D., Cody, E. and Lynn, H. 1975. Blood-feeding activity of partially engorged Culex nigripalpus (Diptera: Culicidae). Entomol. Exp. Applic. 18: 261-268.
Hardy, J. L. 1987. The ecology of western equine encephalomyelitis virus in the central valley of California, 1945—1985. Am. J. Trop. Med. Hyg. 37(Suppl.): 18s-32s.
Henderson, L. P., Brust, R. A. and Wong, F. C. 1979. Biological transmission of western encephalomyelitis virus by Culex tarsalis Coquillett. Mosq. News 39: 385-390.
Holden, P., Hayes, R. O., Mitchell, C. J., Francy, D. B., Lazuick, J. S. and Hughes, T. B. 1973. House sparrows, Passer domesticus (L.) as hosts of arboviruses in Hale County, Texas. I. Field studies, 1965-1969. Am. J. Trop. Med. Hyg. 22: 244-253.
Kale, H. W., Edman, J. D. and Webber, L. A. 1972. Effect of behavior and age of individual ciconiiform birds on mosquito feeding success. Mosq. News 32: 343-350.
Kimsey, R. B. and Kimsey, P. B. 1984. Identification of arthropod blood meals using rubidium as a marker: a preliminary study. J. Med. Entomol. 21: 714-719.
Klowden, M. J. 1988. Factors influencing multiple host contacts by mosquitoes during a single gonotrophic cycle, pp. 29—36. In: T. W. Scott and J. Grumstrup-Scott [eds.], The role of vector-host interactions in disease transmission. Misc. Publ. Entomol. Soc. Am. 68.
Klowden, M. J. and Briegel, H. 1994. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between Anopheles and Aedes (Diptera: Culicidae). J. Med. Entomol. 31: 618-622.
Mitchell, C. J. and Millian, K. Y. Jr. 1981. Continued host seeking by partially engorged Culex tarsalis (Diptera: Culicidae) collected in nature. J. Med. Entomol. 18: 249-250.
Mitchell, C. J., Francy, D. B. and Monath, T. P. 1980. Arthropod vectors, pp. 313—379. In: T. P. Monath [ed.], St. Louis Encephalitis. American Public Health Association, Washington, DC.
Ribeiro, J. M. C. 1987. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 32: 463-478.
Scott, T. W., Chow, E., Strickman, D., Kittayapong, P., Wirtz, R. A., Lorenz, L. H. and Edman, J. D. 1993. Blood-feeding patterns of Aedes aegypti (Diptera:Culicidae) collected in a rural Thai village. J. Med. Entomol. 30: 922-927.
Smith, C. E. G. 1987. Factors influencing the transmission of western equine encephalomyelitis virus between its vertebrate maintenance hosts and from them to humans. Am. J. Trop. Med. Hyg. 37(Suppl.): 33s-39s.
Sokal, R. R. and Rohlf, F. J. 1981. Biometry, 2nd ed. Freeman, New York.
Statistix. 1992. Analytical Software, Tallahassee, Florida, USA.
Trpis, M. and Hausermann, W. 1986. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am. J. Trop. Med. Hyg. 35: 1263-1279.
Washino, R. K. and Tempelis, C. H. 1983. Mosquito host bloodmeal identification. Annu. Rev. Entomol. 28: 179-201.
Weatherhead, P. J. 1981. The dynamics of red-winged blackbird populations at four late summer roosts in Quebec. J. Field Ornithol. 52: 222-227.
Weatherhead, P. J. 1983. Sex ratio adjustment in red-winged blackbirds (Agelaius phoeniceus). Behav. Ecol. Sociobiol. 12: 57-61.
Webber, L. A. and Edman, J. D. 1972. Anti-mosquito behaviour of ciconiiform birds. Anim. Behav. 20: 228-232.